It’s been 10 years since Jenny and Karl Herz started in business. Over the past 10 years they’ve launched Biointelect and Biocelect businesses to help secure approval and distribution for new medicines into Australia.
Biointelect is a strategic planning and commercialisation firm for the biopharmaceutical and medical device sector including commercial, government and not-for-profit organisations.
Biointelect helps its clients develop and drive strategy, identify and evaluate new business opportunities and engage the right partners. The strategy consulting involves therapeutic development from start up through to post launch, with an Australian and a Global perspective.
Biointelect’s mission is to bring science to market.
Biocelect works with local and overseas partners to bring critical pharmaceutical products and medical technology solutions to the Australian market.
In addition to our travel range, infectious disease portfolio and novel medical diagnostics, Biocelect is the sponsor of the Novavax COVID-19 Vaccine, NVX-CoV2373 for Australia and New Zealand.
Biocelect’s mission is to build pathways to patients.
In this Australian Health Journal interview, Jenny and Karl talk about the journey the husband and wife team took to get the Novavax COVID-19 vaccine (Nuvaxovid) approved and distributed in Australia. The journey didn’t just include talented and diverse skilled staff but also their children working in both organisations.
You Might also like
-
Australian Healthcare and Hospitals Association Hospitals and Clinics Innovation New Content Robotics Technology
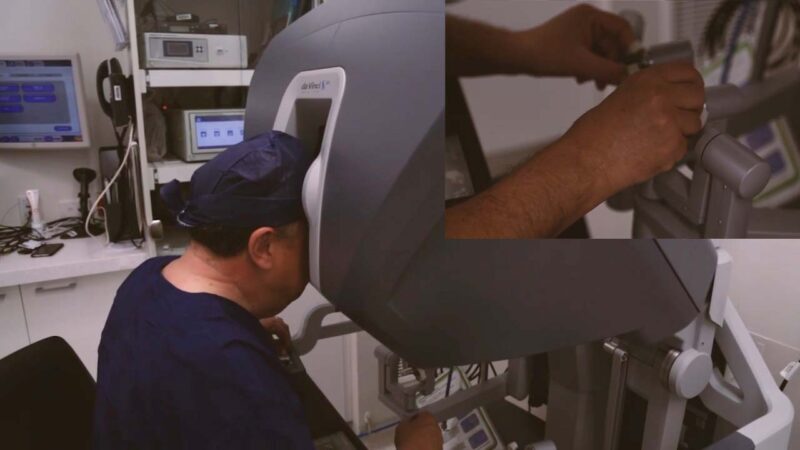
Future of minimally invasive surgery
Macquarie University Hospital is the first hospital in Australia to have three robotic surgical systems. It remains the busiest centre for robotic urology in New South Wales and has rapidly growing programs in other areas. What is behind the Hospital’s success?
Conjoint Associate Professor Walter Kmet, CEO of Macquarie University Hospital, says that the story of robotics at the Hospital is driven by its academic health sciences identity.
-
Clinical research integrates with GP and Pharmacist workflows to supplement practice revenue
Clinical trials are crucial to the development of evidence-based preventative medicines. In addition, participation in clinical trials can also provide patients with opportunities to access new treatments.
“Clinical trials are at the heart of medical advances which look into new ways to treat, prevent, or detect disease. Volunteers often do so to help contribute to advancing scientific research, knowing that they are participating in the hope of helping future generations,” said Charlotte Bradshaw, CEO and Founder to Evrima Technologies.
-
Improved access to technology needed for people with Type 2 Diabetes needing insulin
The National Diabetes Services Scheme (NDSS) provides subsidised products for diabetes management; however, disparities exist in access to technology between those with Type 1 and Type 2 diabetes. While continuous glucose monitoring (CGM) devices are subsidised for Type 1, they are not available for Type 2 diabetes. ADEA advocates for equitable access to these essential tools, emphasising that all individuals with diabetes deserve the resources necessary for optimal management and reduced risk of complications. Without such technologies, many are forced to rely on finger pricking, which can be inconvenient and unsafe in settings such as the workplace and in higher education.